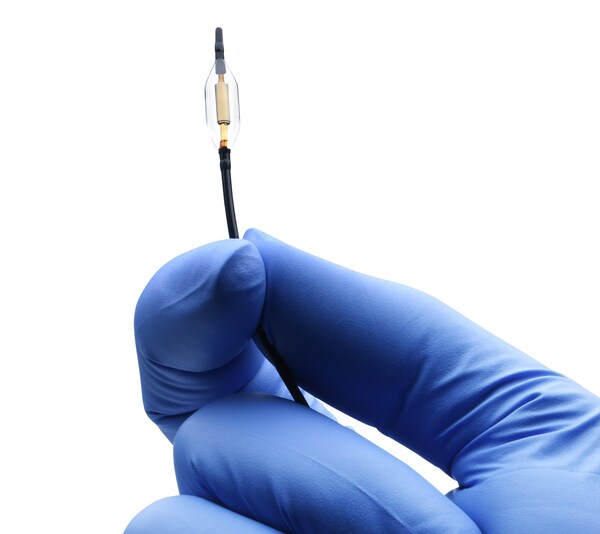
The Paradise Ultrasound Renal Denervation (uRDN) system becomes the first and only ultrasound-based renal denervation (RDN) therapy to be granted a distinct category and device code for incremental Outpatient Transitional Pass-through (TPT) payment by CMS, recognizing the differentiation of Paradise uRDN system in facilitating Medicare fee-for-service beneficiary access to new and innovative medical technologies.
The Paradise Ultrasound Renal Denervation System
CMS Grants Transitional Pass-Through (TPT) Payment for Recor’s Paradise Ultrasound Renal Denervation System
Palo Alto, CA, Nov. 01, 2024 (GLOBE NEWSWIRE) — Recor Medical, Inc. (“Recor”) and its parent company, Otsuka Medical Devices Co., Ltd. (“Otsuka Medical Devices”) today announced U.S. Centers for Medicare & Medicaid Services (CMS) have granted the company’s Paradise® Ultrasound Renal Denervation system a Transitional Pass-through (TPT) payment. The approval of TPT offers incremental reimbursement payments for outpatient procedures performed with ultrasound renal denervation for Medicare fee-for-service beneficiaries. It becomes effective January 1, 2025, and is expected to remain effective for up to three years.
In approving the TPT, CMS created a distinct device category and code (C1736: Catheter Renal Denervation, Ultrasound) for Ultrasound Renal Denervation in recognition of the differentiated technology and procedure with the Paradise uRDN System. Additional details and the notice can be found here: https://public-inspection.federalregister.gov/2024-25521.pdf
The Paradise uRDN system is a first-of-its-kind ultrasound-based RDN technology designed to lower blood pressure by denervating the sympathetic nerves surrounding the renal arteries, reducing the overactivity that can lead to hypertension. The Paradise uRDN system delivers two to three doses of 360-degree ultrasound energy — lasting seven seconds each — through the main renal arteries to the surrounding nerves. The Paradise catheter features the exclusive HydroCooling™ system, which circulates sterile water through the balloon catheter during the procedure to help protect the renal artery wall.
“TPT for ultrasound renal denervation increases access to a proven device-based hypertension treatment option to patients who have been unable to achieve blood pressure control with lifestyle changes and medications alone,” said Lara Barghout, president and CEO of Recor Medical. “The granting of TPT highlights the safety and efficacy of this breakthrough device, which together demonstrated that the Paradise uRDN system met the newness and significant clinical improvement criteria. By creating a distinct Device Category, CMS have also recognized that the Paradise uRDN system is a highly differentiated technology and that there are significant differences in comparison to other technologies available in the marketplace. This is a major step forward in the reimbursement available for the Paradise uRDN system, creating additional financial support for hospitals and physicians to provide this novel and effective therapy to their uncontrolled hypertension patients.”
Hypertension, or high blood pressure, is the leading contributor to disease burden worldwide, resulting in increased cardiovascular morbidity and mortality, poor quality of life, and higher costs to health systems. The U.S. Food and Drug Administration (FDA) approved Recor’s Paradise Ultrasound Renal Denervation system for the treatment of hypertension on November 7, 2023. The Paradise uRDN system is intended as an adjunctive treatment option when lifestyle changes and medications have not adequately controlled a patient’s blood pressure.
About Recor Medical, Inc.
Recor Medical, headquartered in Palo Alto, Calif., a wholly owned subsidiary of Otsuka Medical Devices Co., Ltd., is a medical technology company focused on transforming the management of hypertension. Recor has pioneered the use of the Paradise Ultrasound Renal Denervation system for the treatment of hypertension. The Paradise uRDN system is an investigational device in Japan, is FDA approved in the United States, and bears the CE mark. Recor has reported positive outcomes in three independent, randomized, sham-controlled studies of the Paradise uRDN system in patients with mild-to-moderate and resistant hypertension. In addition, Recor is conducting the Global Paradise System (“GPS”) Registry in the European Union and the UK, and has initiated the US GPS post-approval study in the United States.
About Otsuka Medical Devices Co., Ltd.
Otsuka Medical Devices Co., Ltd. engages in the global development and commercialization of medical devices that provide new therapeutic options in areas where patient needs cannot be met through pharmaceutical or other conventional treatment. Otsuka Medical Devices is a subsidiary of Otsuka Holdings Co., Ltd. (www.otsuka.com/en), a global healthcare company listed on the Tokyo Stock Exchange (JP 4578).
https://www.omd.otsuka.com/en/
Media Contact
Lisa Owens
The Mullings Group
lowens@mullingsgroup.com
+1-210-601-6647
Attachments
- The Paradise Ultrasound Renal Denervation System
- CMS Grants Transitional Pass-Through (TPT) Payment for Recor’s Paradise Ultrasound Renal Denervation System
CONTACT: Lara Barghout Recor Medical +1 650 542 7700 lara.barghout@recormedical.com